Heterogeneous and Homogeneous Mixture
What is a mixture?
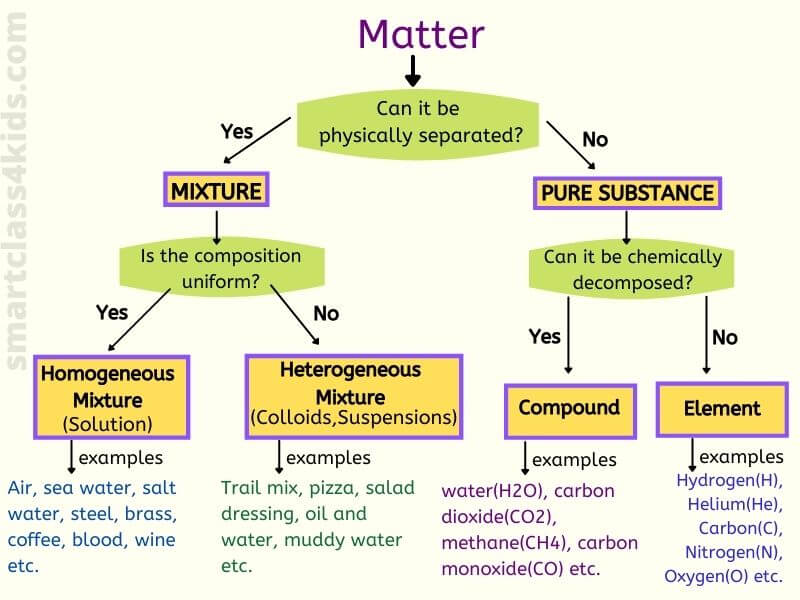
A mixture is a matter that consists of TWO or more types of substances mixed PHYSICALLY but NOT chemically combined. Since mixtures are not chemically combined, we can separate them by physical methods. Mixtures can be solid, liquid, gas.
Also Read: Classifying Matter
Properties of mixture
There are 5 properties of mixtures listed below.- The mixture has no fixed proportion of the components.
- The substances of a mixture retain their properties.
- The mixture can be separated by physical methods based on the physical properties of substances.
- No chemical process is involved in the formation of a mixture.
- The mixture has no fixed melting points and boiling points.
Examples of a mixture
- Soda: a mixture of water, sugar, and flavorings.
- Fog – a mixture of water droplets and air
- Blood: A mixture of white blood cells, red blood cells, plasma and platelets
- Gasoline – a mixture of Petroleum, hydrocarbons, and fuel additives
- Crude oil: A mixture of organic compounds (mainly hydrocarbons)
- Seawater: A mixture of various salt and water.
- Air: a mixture of different gases like oxygen, carbon dioxide, nitrogen, argon, etc.
- Bleach: A mixture of chlorine, caustic soda, and water.
- Gunpowder: A mixture of sulfur, potassium nitrate, and carbon.
Types of mixture
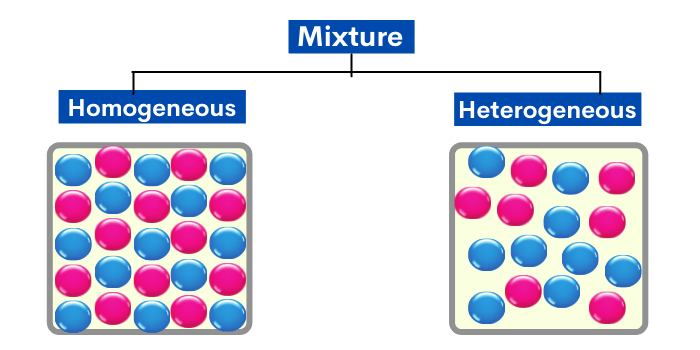
What is a homogeneous mixture?
“Homo” is a Greek word that means “same,” “genus” means “kind.”
In this type of mixture, substances are so evenly mixed that it is difficult to distinguish one substance from another. It appears to contain only one kind of substance.
In simple words, If you can not see the different substances in a mixture, it’s called homogeneous.
Types of homogeneous mixture?
- Solutions: A mixture formed when a substance (the solute) is dissolved in another substance (the solvent).
- Solute: the part of a solution that is being dissolved (usually the lesser part).
Solvent: the part of a solution that dissolves the solute (usually the greater part).SOLUTION = SOLVENT + SOLUTE
Examples of homogeneous mixture
- A glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample. You can’t easily separate the lemon juice from the water; it’s uniformly mixed.
- Stainless steel is made from a homogeneous mixture of iron, chromium, and nickel.
- Air is a homogeneous mixture of nitrogen gas (78%), oxygen gas (21%), and small amounts of various other gases.
- Oil, vinegar, dishwashing liquid, brass, wine, blood, seawater, natural gas, etc.
What is a heterogeneous mixture?
“Hetero” is a Greek word that means “different,” “genus” means “kind.”
In a heterogeneous mixture, substances are NOT evenly mixed. The parts of these mixtures are noticeably different from one another.
In simple words, If you can see the different substances in a mixture, it’s called heterogeneous.
Types of heterogeneous mixture?
- Suspensions: a heterogeneous mixture in which the solute particles are so large that they settle out when left undisturbed for sometime. The particles of a suspension are visible to the naked eye. When suspension are stirred, the particles seems to be evenly distibuted throughout the mixture. e.g. sand-water, oil-water, dust particles in air etc.
- Colloids: a mixture in which the dispersed particles are medium in size between those of a solution and a suspension. It means colloid is the intermediate state between solution and suspension. E.g. paints, milk, butter, fog etc.
Examples of a heterogeneous mixture
- sand and sugar
- salt and gravel
- ice cubes in a drink
- sand and water
- salt and oil
- oil and water
- orange juice with pulp
- chicken noodle soup
- cereal and milk
- oatmeal and raisins
- salad
Characteristics of mixture
The major characteristics of mixtures are discussed below.- Mixtures may be solid, liquid, or gas.
- Mixtures are impure substances.
- The constituents of a mixture do not exist in a fixed ratio.
- A mixture is a combination of two or more substances that are NOT chemically combined.
- The substances may be separated by physical methods such as filtration, freezing, and distillation.
- They can either be heterogeneous or homogeneous in nature.
- There is no change in energy during the formation of a mixture (energy is neither produced nor evolved).
- The mixture retains the properties of its components.
Difference between homogeneous and heterogeneous mixture
Homogeneous
- mixed evenly
- These are called solutions
- Constituent particles are not visible to the naked eye
- Separated chemically
- They represent the same physical properties
- Homogeneous mixture examples are milk, oil, fog, seawater, etc.
Heterogeneous
- mixed unevenly
- These are called suspension/colloids
- Constituent particles are easily visible to the naked eye
- Separated physically
- They do not possess same physical properties
- Heterogeneous mixture examples are sand water, salad, pizza, water-oil, salt-pepper, etc.
Is water a mixture?
No, water is not a mixture. Water is a pure substance because it only contains one type of molecule, i.e., H2O. Another point to think about is that a mixture is produced when two substances are physically combined, not chemically. What would happen if you release hydrogen and oxygen from its cylinder in the environment? Do you get water? No! You don’t get water because you just mix hydrogen and oxygen molecules physically.
But if you add enough energy to the mixture of hydrogen and oxygen, then a chemical reaction occurs, and this way, water can be formed. So you can say that water is a pure substance and not a mixture.
Is air a mixture?
Yes, the air is a mixture because air can be separated into its constituents like nitrogen, oxygen etc., by the physical process of fractional distillation. Air has a variable composition, and it does not have a fixed formula well.Is air a homogeneous mixture?
Yes, the air is a homogeneous mixture of nitrogen gas (78%), oxygen gas (21%), and small amounts of various other gases. It means, In the air, all gases would have a uniform composition. Therefore, the air is a homogeneous mixture.
Is soil a mixture?
Yes, the soil is a mixture of sand, clay, minerals, gases, liquids, and organic substances like dead plant material and water.Is rocky road ice cream a mixture?
Yes, a rocky road ice cream is an example of a heterogeneous mixture.Is vinegar a mixture?
Yes, vinegar is a homogenous mixture of acetic acid and water. As the mixture created has only one phase, it is a solution. Through combining two or more chemical substances, mixtures are produced. If the result has more than one phase, it is a mixture; else, it is called a solution.
Is cough syrup a mixture?
Yes, cough syrup is an example of a homogeneous mixture.Related Topics:
State of Matter
Physical Properties of Matter
Classifying Matter
Changing States of Matter
Oxygen Facts
Chemical & Physical Change
Force
How Do Magnet Works
I hope you have understood the concept of physical properties of matter well. Don’t forget to attempt the properties of matter quiz.
