Physical Properties of Matter
- The matter is everything around you.
- The matter is anything that has VOLUME and MASS.
- Matter is made up of extremely tiny particles called atoms and molecules.
- An atom is composed of electrons, protons, and neutrons.
- States of matter: Solid, Liquid, Gas, Plasmas, and Bose-Einstein condensates (BEC).
- Physical Properties of Matter: color, shape, texture, odor, taste, malleability, conductivity, elasticity, magnetism, relative density, mass, opacity, volatility, diffusion, transparency and solubility.
- For detail, “State of Matter.”
All matter has physical and chemical properties. A property is a characteristic or feature of an object that distinguishes one substance from another.
Matter can be classified by its physical properties and chemical properties.
A physical property can be measured or observed without changing the identity of the substance.
A change in the size, shape or state of matter is called a physical change.
In a chemical change, bonding patterns of matter change and new substances form. All chemical reactions are considered chemical changes.
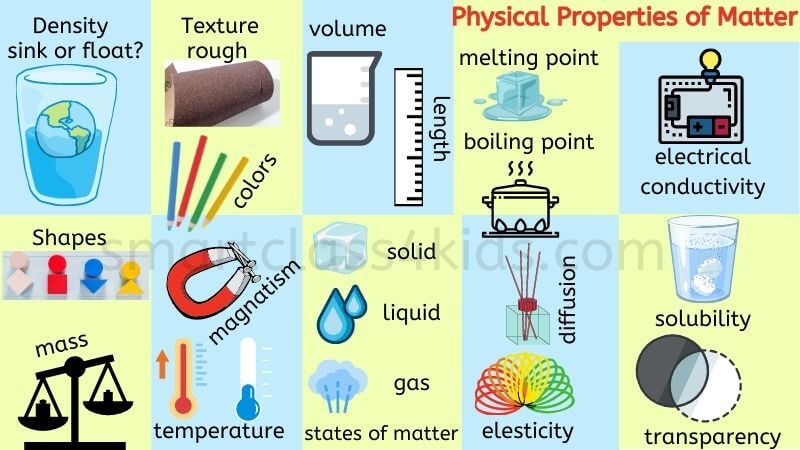
There are two types of physical properties: Extensive and Intensive physical properties.
Extensive physical properties: An extensive property is a physical property of matter that depends on the amount of matter. Extensive properties including:
- Mass
- Volume
- Length
- Size
- Shape.
Question for you: Consider two situations, one in which an object of mass M is on the Earth and second in which the same object of mass M is on the moon. Will the mass and weight of the object be the same at both places?
Answer: NO, mass and weight will not be the same at both places. Weight will change, but the mass of an object will stay the same.
Reason: The amount of matter (mass) present in the object is not changing, no matter where it is present.
For example, a person with a mass of 60 kg on Earth will have the same mass of 60 kg in space or on the moon. But the person’s weight would not be the same in those places. The weight would be only one-sixth on the moon compared to the Earth because the moon has less gravity than earth.
But it doesn’t mean that the object becomes smaller physically on the moon. The mass of an object stays the same, but because of gravity, the object’s weight changes.
Let’s understand why the weight is different on the Earth and the moon.
Weight is the measurement of the force of gravity pulling on an object. So weight can change depending on the gravity field. Things weighed less on the moon than on the Earth because the moon has smaller gravity than the Earth, but the mass is absolutely the same.
“When the properties of a substance are measured with an instrument such as a ruler, beaker, graduated cylinder, scale, etc., it is called measurable properties. Mass, weight, volume, density are considered measurable properties of matter.”
“When we describe the properties of a substance using our five senses, it is called an observable physical property. Malleability, color, odor, texture, hardness, conductivity, elasticity, ductility, solubility, state of matter, magnetism etc., are considered as observable physical properties of matter.”
Mass: The mass is the amount of matter in an object. Mass is measured in milligrams (mg), grams (g), or kilograms (kg).
“Mass is a measure of how much matter an object contains”.
Mass is proportional to weight. It means the more mass an object has, the more weight it has. Mass and weight depend on each other.
The standard metric unit (SI) of mass is the kilogram (kg).
Weight: It is the measurement of how strongly gravitational force pulls on an object. This gravitational force is directly proportional to the mass of the object. It means, once the force of gravity changes, the weight of an object changes, but its mass remains the same.
“Weight is a measure of the force of gravity on the object”.
What is gravity: Gravity is the force that pulls objects towards the core of the Earth.
Have you ever thought about why objects with more mass usually weigh more than objects with less mass? Because gravity pulls with greater force on objects with greater mass.
Volume: Volume is the total amount of space occupied by a substance.
The volume of liquids is measured using either a graduated cylinder or a buret.
The volume of gases depends on the volume of their container.
The volume of regularly shaped solids can be calculated from their three dimensions. Volume = length × width × height.
Law of Conservation of Matter
According to this law, matter can neither be created nor destroyed. Substances change from one form to another, but the total mass remains the same. In a chemical change, the total mass of the reactants and the products always remains the same.
Test your knowledge
- Put one ice cube in a ziplock bag and measure the mass of the ice cube with the bag.
- Wait until the ice cube melts. Once it melts completely, measure the ice cube with the bag once again.
- Compare the mass of the ziplock bag before and after the ice cube melted.
Intensive physical properties: If the properties do NOT depend on the amount of matter, called intensive physical properties. Examples of intensive properties include:
- Color
- Odor
- State of Matter
- Texture
- Hardness
- Magnetism
- Density
- Malleability
- Ductility
- Elasticity
- Solubility
- Conductivity
- Volatility
- Diffusion
- Transparency
- Boiling Point
- Melting Point
Color: We can classify the objects on the basis of their color. Water and alcohol are colorless, but petrol and diesel are colored liquid. Sulfur is yellow, and copper is red in color.
Odor: Odor or smell is considered to be an intensive physical property because the smell of any substance does not depend on its quantity. Whether the object is more or less, its smell will be the same.
The smell emanating from an object can also be helpful in classifying a substance. For example, petrol or diesel can be distinguished from water by their odor. Vinegar has a very acidic odor, chlorine has a very strong bleach smell, and oxygen and nitrogen are odorless.
State of Matter: Matter can either be a solid, liquid, gas, or plasma.
Texture: Texture is the feel or appearance of a surface. Some textures that objects can have are soft, hard, smooth, rough, bumpy, silky, sticky, and chalky.
Hardness: Hardness describes an object’s resistance to being scratched or dented. We can compare the hardness of an object by rubbing it against another object.
For example, what will happen if we rub the glass with a stone? Obviously, there will be scratches on the glass.
But what if we rub the glass with talcum powder instead of stone? Then there will be no scratch at all. This is because a stone is harder than glass, and talcum powder is softer than glass.
Magnetism: Magnetism is a physical property of some metals such as iron, cobalt, and nickel. Objects that get attracted to a magnet are called magnetic objects, and the objects that don’t get attracted to a magnet are called Non-Magnetic objects.
For example, aluminum, copper, silver, gold, plastic, wood, cloth etc., are not attracted by magnets.

Density: Density is a measure of how compact the mass is compared to its size (volume). Volume is the amount of space an object occupies.
Relative density is when we compare the density of one thing to the density of another.
Usually, we compare the density of an object with the density of water.
The density of an object v/s density of water.
If an object is denser than water, it will sink when placed in water.
If an object is less dense than water, it will float.
Dense means how closely packed the particles are in an object.

The density of plastic duck < density of wooden ball < density of iron ball.
Density also depends on the material. A piece of iron with the same dimensions as a plastic toy duck will be heavier because the atoms are more closely packed, and each iron atom has much more mass than each plastic atom. That is why the plastic toy duck floats and the iron piece sinks when placed in water.
How to measure the density of solid?
Density = mass / volume or, D= m/v
TRY IT OUT: A block of wood has a mass of 6 g and occupies a volume of 12 cm³. What is its density?


Elasticity: Some objects change their shape and size temporarily by applying a force on them; they are known as elastic objects. For example, When we stretch the rubber or spring, it changes its shape and size but as soon as we release it, they regain their original form. This is because rubber and spring have the property of elasticity.
Solubility: Solubility is the ability of a substance to dissolve in a liquid. Some substances like sugar, salt etc., completely dissolve in water, they are classified as water-soluble, and this property is called solubility.

Conductivity: Conductivity refers to how easily a substance conducts electricity or how well it can transmit heat.
Conductors allow energy to pass.
Insulators slow energy transfer.
Materials like copper, aluminum, iron in which heat or electric current flows easily and quickly are called good conductors of both heat and electrical energy.
Materials like air, wood, glass are examples of bad conductors because these materials do not conduct or transfer the flow of heat or electricity (or both).
Examples of good conductors of heat: silver, copper, aluminum, brass, iron, lead, stainless steel etc.
Examples of bad conductors of heat: air, water, cork, glass, styrofoam etc.
Examples of good conductors of electricity: silver, gold, copper, steel, seawater, platinum etc.
Examples of bad conductors of electricity: rubber, glass, oil, diamond, dry wood etc.

Volatility: Volatility is a physical property of matter which describes how easily a substance vaporizes. For example, petrol, alcohol, spirit, diesel, etc., are volatile substances. Mercury is also a volatile element.
Diffusion: Diffusion is the movement of molecules of matter from high concentration towards low concentration.
For example, when we spray room freshener in one corner of the room, it spreads to the rest through diffusion.
A drop of food coloring in a glass of water gives color to the water through diffusion.
Transparency: Transparency is the physical property of letting light pass through the object without an appreciable scattering of light. For example, glass, clean water etc., are transparent materials.
Boiling Point: The temperature at which a substance starts boiling. At this point in temperature, a liquid turns into a gas. Water boils at 100 °C (212 °F), so we can say that the boiling point of water is 100 °C (212 °F).
Melting Point: The temperature at which a substance starts melting. At this point in temperature, a solid turns into a liquid. The melting point of pure water ice is 32°F (0°C).
Physical Properties of Matter: In Summary
Matter: Matter is anything that has mass and takes up space (volume).
Physical Properties: A physical property can be observed or measured with our 5 senses without changing the identity of the substance.
Intensive property: Any characteristic of matter that does not depend on the amount of the substance present.
Extensive property: Any characteristic of matter that depends on the amount of matter being measured.
Mass: The amount of matter in an object.
State of Matter: A form that matter can take: solid, liquid or gas.
Texture: the visual and tactile quality of a surface.
Hardness: the measure of the resistance of a solid to being scratched or dented.
Magnetism: a force that pull magnetic objects.
Density: Whether something sinks or floats in liquid.
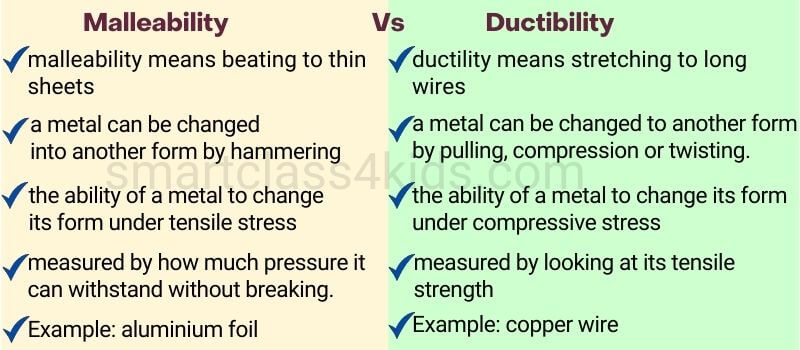
Malleability: capable of being flattened into thin sheets by hammering.
Ductility: the ability to be drawn or pulled into a wire.
Elasticity: ability to resist a distorting influence and to regain its original size and shape when that force is removed.
Solubility: Ability of a solid to dissolve in a liquid.
Conductivity: Allows electricity or heat to flow through it.
Volatility: describes how easily a substance will vaporize (turn into a gas or vapor).
Diffusion: movement of molecules of matter from high concentration towards the low concentration.
Transparency: allowing light to pass through the material.
Boiling Point: The temperature at which a substance starts boiling.
Melting Point: The temperature at which a substance starts melting.
Now It's Your Turn

Related Topics:
State of Matter
Physical Properties of Matter
Classifying Matter
Changing States of Matter
Oxygen Facts
Chemical & Physical Change
Force
How Do Magnet Works
I hope you have understood the concept of physical properties of matter well. Don’t forget to attempt the properties of matter quiz.
