Changing States of Matter
What is changing states of matter?
Materials can be changed from one state to another by heating or cooling (freezing). By adding heat energy, a substance can change from a solid to a liquid or from a liquid to a gas. So the state of a matter depends on its gaining or losing heat energy.
Water is a good example of the state of matter because it’s three states are all pretty common.
The solid form of water is ice.
The liquid form of water is water.
The gas form of water is steam.

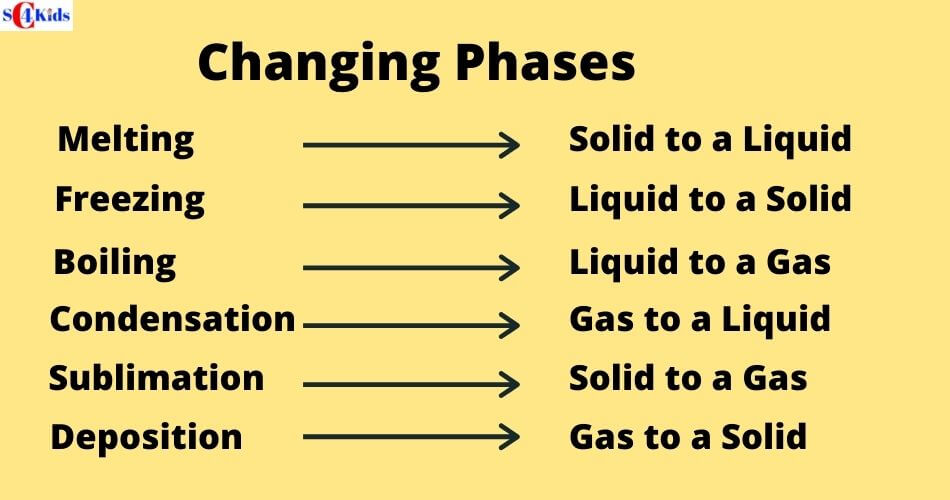
Melting: Solid to Liquid
Melting is a process where a solid changes to a liquid by adding heat.
Melting occurs when the heat energy breaks the bonds (attractions) between the molecules and allows them to move past each other as a liquid. As a result, the liquid started flowing.
The temperature at which a solid becomes a liquid is called its MELTING POINT. The melting point of water is 0 degrees C (32 degrees F).
Freezing: Liquid to Solid
Freezing is a process where a liquid changes to a solid by cooling.
If water (liquid state) is cooled, it changes to ice (solid-state). This change is called FREEZING. The temperature at which this occurs is called the freezing point (fp) of the substance. Water freezes at 0°C.
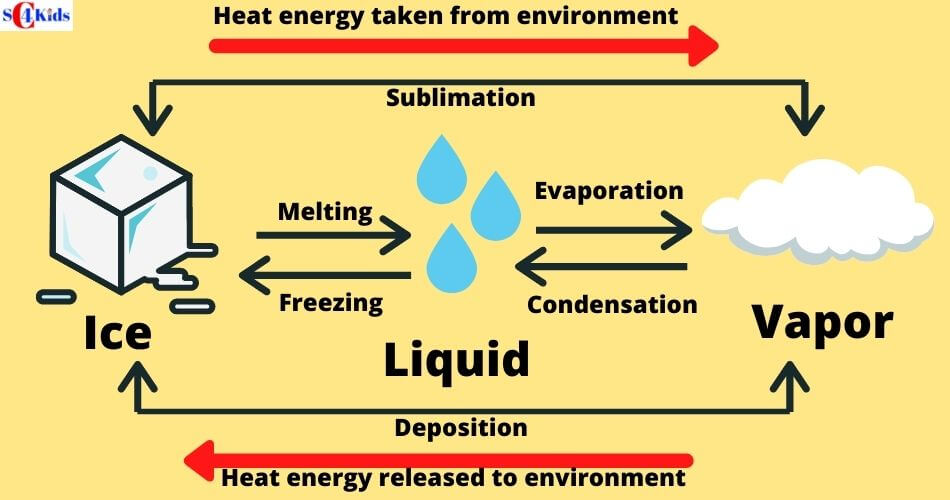
Evaporation/Boiling: Liquid to Gas
Evaporation is a process where a liquid changes to gas by adding heat.
If water is heated to 100 degrees C, the molecules move faster and start to break free, flowing as a gas. The temperature necessary to reach this point is called the BOILING POINT.
Condensation: Gas to Liquid
Condensation is the process where a gas changes to a liquid by cooling.
When a gas cools, the molecules of gas lose energy and stick together, turning the gas into a liquid. The temperature at which this transformation occurs is known as the CONDENSATION POINT. Condensation causes rain, fog, mist, etc.
Sublimation: Solid to Gas
Sublimation is a process where solid transforms into a gas without ever becoming a liquid phase. When dry ice gets exposed to air, dry ice directly changes its phase from solid to gas which is visible as fog.
Sublimation occurs when solid absorbs energy so quickly from the surroundings that melting never occurs.
Examples of Sublimation:
- Dry ice
- Moth balls sublime
- Frozen foods will sublime and you will find ice crystals inside of the box or bag.
Frequently Asked Questions (FAQs)
How many changes of state are there?
There are six changes of phase: freezing, melting, condensation, boiling, sublimation and deposition
What's it called when a solid turns into a gas?
The solid-to-gas change is called sublimation, while the reverse process is called deposition.
What does vaporisation mean?
Vaporization of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling.
Can a solid go straight to a gas?
Yes, Sublimation is the process of transformation directly from the solid phase to the gaseous phase, without passing through an intermediate liquid phase
