Classifying Matter
- The matter is everything around you.
- The matter is anything that has VOLUME and MASS.
- Matter is made up of extremely tiny particles called atoms and molecules.
- An atom is composed of electrons, protons, and neutrons.
- States of matter: Solid, Liquid, Gas, Plasmas, and Bose-Einstein condensates (BEC).
- Physical Properties of Matter: color, shape, texture, odor, taste, malleability, conductivity, elasticity, magnetism, relative density, mass, opacity, volatility, diffusion, transparency and solubility.
- For detail, “State of Matter.”
Classifying Matter
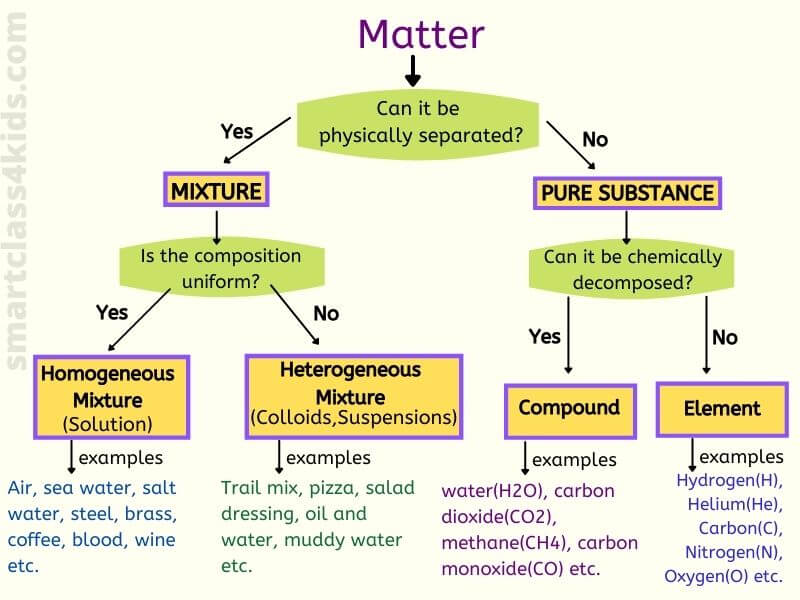
Pure Substance
A pure substance is a matter that is made of only one type of atom or only one type of molecule (a molecule is a group of atoms bonded together).
For example, copper wire is considered a pure substance because it contains only copper atoms. No matter how small you break the copper wire, its particles will remain the same.

Water is also considered a pure substance because every sample of water would contain a molecule of water (H2O); no matter how they are prepared, they will always have the same ratio of atoms, i.e., two atoms of hydrogen and one atom of oxygen.
Pure substances can be classified into two categories: Elements and Compounds.
Elements
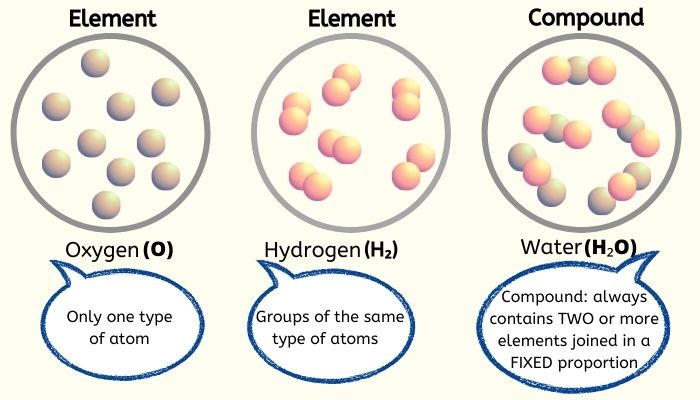
An element is a substance that contains only one type of atom. It cannot be separated or broken down into simpler substances by physical or chemical changes.
Element is also known as the “simplest form or the basic form of a pure substance.“
For example, Gold is considered an element because no matter how small we break Gold into pieces, its particles will remain exactly the same, i.e., gold atoms.
Iron, aluminum, copper, silver, phosphorus, sulfur, oxygen, hydrogen, etc., are examples of elements.
There are 118 elements on the periodic table.
Compounds
A compound is a pure substance that contains two or more types of atoms or elements that are chemically joined together in a fixed proportion.
Compounds can be broken down into elements through chemical change. A chemical change is a change that forms one or more new substances.
For example, Carbon dioxide (CO2) is also a compound. It consists of atoms of two elements, Carbon (Solid) & Oxygen (Gas). When they are chemically combined, they form the compound Carbon dioxide (Gas).
A compound often has different properties from the individual elements that comprise it.
Table salt is another example of a chemical compound. Two elements, sodium (Na) and Chloride (Cl) combine to form table salt (NaCl).

Mixture
A mixture is a matter that consists of TWO or more substances mixed together PHYSICALLY but NOT chemically combined. Since mixtures are not chemically combined, we can separate them by physical methods.
- The mixture contains two or more substances.
- Substances retain their own properties.
- Substances may change in physical appearance.
- The amount of substance can vary in different parts of the mixture.
- Mixtures are classified further by how well the amount of substance is distributed.
- There are two main types of mixtures: homogeneous mixture and Heterogeneous mixtures.
- The mixture can be separated by physical means based on the physical properties of substances.
- Methods of mixtures separation are: sedimentation, decantation, filtration, evaporation, crystallization and distillation etc.
Homogeneous Mixture:
“Homo” is a Greek word that means “same,” “genus” means “kind.” In this type of mixture, substances are so evenly mixed that it is difficult to distinguish one substance in the mixture from another.
It appears to contain only one type of substance.
For example, a glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample.
Stainless steel is made from a homogeneous mixture of iron, chromium, and nickel.
Air is a homogeneous mixture of nitrogen gas (78%), oxygen gas (21%), and small amounts of various other gases.
A homogeneous mixture is also known as SOLUTION.
SOLUTE: the part of a solution that is being dissolved (usually the lesser part).
SOLVENT: the part of a solution that dissolves the solute (usually the greater part).
SOLUTION = SOLVENT + SOLUTE
Example: In a solution of saltwater,
Water is solvent,
Salt is solute.

Heterogeneous Mixture:
“Hetero” is a Greek word that means “different,” “genus” means “kind.”
In a heterogeneous mixture, substances are NOT evenly mixed. The parts of these mixtures are noticeably different from one another.
Examples:
Sand and water
Oil and water
Salt and pepper
If we take two samples from a mixture of sand and water, the proportions of the components in each sample will be different.
Difference between Compounds & Mixtures
Compounds
➤ Compound is a pure substance made up of two or more elements combined chemically. E.g. H2 + O = H2O (water).
➤ Particles lose their original properties. H2 (gas) + O (gas)= H2O (liquid)
➤ Compounds are homogeneous.
➤ Compounds can not be separated by physical method. Can you separate hydrogen and oxygen from a glass of water?
➤ Every compound has its own chemical formula. H2O (water), CO2 (carbon dioxide),
➤ Compounds have fixed composition by mass. H2O (2 atoms of hydrogen and one oxygen) = water. H2O2 (2 atoms of hydrogen and two atoms of oxygen) = hydrogen peroxide.
➤ A compound has a fixed melting point and boiling point.
➤ Examples: water, carbon dioxide, sodium chloride etc.
Mixtures
➤ A mixture is not a pure substance, made up of two or more substances mixed physically. E.g. NaCl (salt) + H2O (water) = saltwater.
➤ Particles retain their original properties. Sand (solid)+ Water(liquid) = Sandwater (solid+liquid)
➤ Mixtures may be homogeneous or heterogeneous.
➤ Compounds can be separated by physical method. You can separate pebbles and water easily from a pebble-water mixture.
➤ Mixtures have no chemical formula.
➤ Compounds have not fixed composition by mass.
Salt (less) + water = saltwater
Salt (more) + water = saltwater
➤ A mixture does not have a fixed melting point and boiling point.
➤ Examples: air, lemonade, salad dressing, sand-water etc.
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture.

- When you mix your favourite cereals in milk for breakfast.
- Filtered tea
- freshly squeezed orange juice
- Table sugar
- Iron
- Salad dressing
- Water
- Gold
- Table salt
- Oxygen
Hints (to distinguish pure substance, element, compound, mixture, homogeneous mixture, heterogeneous mixture) :
- Decide whether a substance is chemically pure. Is it made of only one type of atom or molecule? If YES, the substance is either an element or a compound.
- Can it be separated into its elements, if YES, it is a compound otherwise it is an element.
- If a substance is NOT chemically pure, it is either a heterogeneous mixture or a homogeneous mixture.
- If its composition is uniform throughout, it is a homogeneous mixture, otherwise it is a heterogeneous mixture.
Answer: 1. heterogeneous mixture, 2.homogeneous mixture 3.heterogeneous mixture 4. Compound 5. Element 6. heterogeneous mixture 7. Compound 8. Element 9. Compound 10. element
Related Topics:
State of Matter
Changing States of Matter
Oxygen Facts
Chemical & Physical Change
I hope you have understood the concept of classification of matter well. Don’t forget to attempt the classifying matter quiz.
